Two years after the rampant and dangerous COVID-19 disease had spread across the globe, the virus continued to mutate into newer variants as different health institutions are researching for vaccines to fight it. Consequently, people have gone through the new normal changes and switches of health restrictions, from the strictest to more lenient today.
Everyone may have been used to what is happening, yet it is undeniable that this virus is still a threat to us. The World Health Organization (WHO) has declared Omicron, the recent SARS-CoV-2 variant, as a variant of concern because of its rapid transmissibility and pathogenicity.
Officially named B.1.1.529 or more commonly known as Omicron, was first identified in Botswana and South Africa in November last year and was named after where its first case was discovered.
Researchers have also learned that Omicron is a variant with more than fifty other variations that include the older discovered Alpha and Beta, and has been eyed to trigger more serious illness to victims.
As of today, the Omicron variant has spread into more than 110 countries worldwide and might be a cause of another outbreak this year because of its dramatic and rapid increase in positive cases. Moreover, Omicron is more transmittable compared to the dangerous Delta variant, resulting in a large number of infections in one area, especially in hospitals.
The early symptoms of Omicron include runny nose, sneezing, headache, fatigue, and sore throat but may appear less concerning than the other variants discovered earlier. Still, because of its neutralizing antibodies, the public is advised to be wary of the said variant. Even with vaccination shots and boosters, one can still catch Omicron if they are not careful enough.
Accordingly, Omicron’s fast communicability raised questions about the effectiveness of rapid antigen tests for detecting the variant.
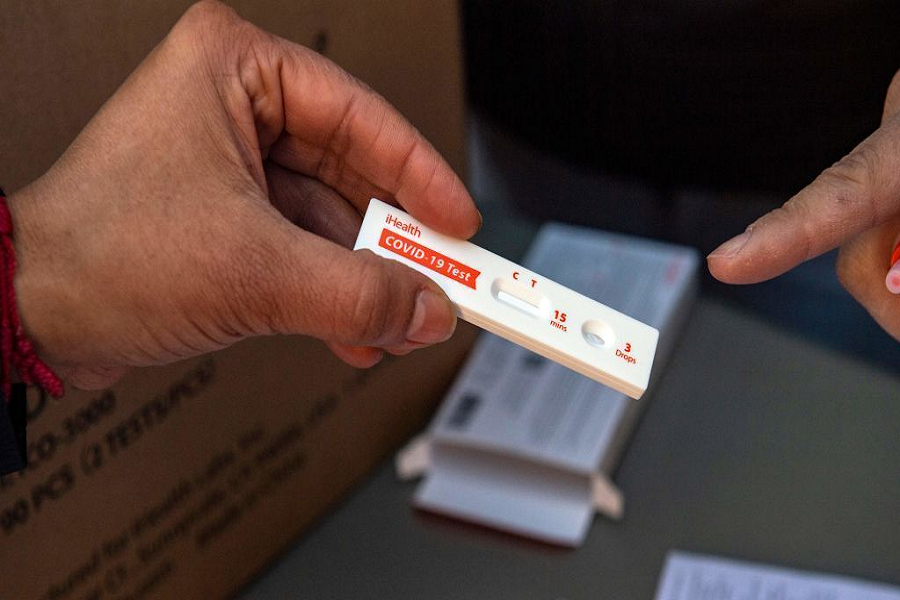
Antigen tests are commonly used to diagnose other respiratory pathogens, including influenza viruses and respiratory syncytial virus or RSV. Because of the unexpected, heavy impact of the sudden surge of COVID-19 back in the last quarter of 2019, the US Food and Drug Administration (FDA) has given its permission for emergency use authorization (EUA) for antigen tests against SARS-CoV-2 identification.
These rapid tests are immunoassays that detect the presence of any specific viral antigen that indicates current viral infection. Furthermore, these are currently authorized to be performed on the nasopharyngeal swab, nasal swab, or saliva specimens that are placed into the assay’s extraction buffer or reagent. Today, there are three types of authorized rapid antigen tests: point-of-care, laboratory-based, and self-tests.
As its name suggests, rapid antigen tests produce quick results, approximately within 15-30 minutes after the procedure. But, if the result takes more than that to be released, a positive case is likely to show.
Rapid antigen tests are used by most nations not only for fast detection of COVID-19 infection but also for fit to fly COVID-19 test. Countries like the UK require travelers to present fit-to-fly certificates that show they do not have the virus and are safe to travel to domestic and international destinations.
Yet because of the continuing mutation and evolution of the COVID-19 virus, the use and effectiveness of rapid antigen tests are still in question. It is still highly suggested that people should stay in the comfort of their homes and only go outside if needed.
To learn more about the reliability of COVID-19 rapid antigen tests, read the infographic from Harley Medic International provided below. Moreover, for an affordable COVID-19 testing kit, contact the trusted and reliable “Official Rapid Tests” by “Harley Medic International.” Get in touch with them through their website.